NOTE: the same discussion, with a better notation and extended to cover the activation energy for oxygen migration, is at 2000-08-10 (section 17)
Mean field analysis for the
![]() reduction in
reduction in
![]() systems.
systems.
Let's take the three cation system
![]() , in which
, in which ![]() is a
three or two valent dopant (valence
is a
three or two valent dopant (valence![]() ). Due to the presence of
the low valent dopant, the structure will contain a number of
compensating oxygen vacancies.
). Due to the presence of
the low valent dopant, the structure will contain a number of
compensating oxygen vacancies.
The mean field picture of this fluorite structured mixed oxide is as follows.
The cationic sites are occupied by a hybrid cationic species ![]() , built up
with a ``linear combination'' of the three cations. In particular, the
charge of
, built up
with a ``linear combination'' of the three cations. In particular, the
charge of ![]() is less than
is less than ![]() because of the smaller
contribution of
because of the smaller
contribution of ![]() ; in fact, the charge of
; in fact, the charge of ![]() is:
is:
As far the anionic sites, the mean field approach does not consider them only partially occupied (as they effectively are); instead, they are all occupied by (let's call them) ``mean field'' oxide ions bearing a smaller negative charge. This charge is given by:
i.e. the charge of a ``regular'' oxide ion corrected for the presence
of the low valent dopant ![]() .
.
If we put a pure ![]() cation at a cationic site, we create a
charged defect; the charge of the defect will be the difference
between the charge of a pure
cation at a cationic site, we create a
charged defect; the charge of the defect will be the difference
between the charge of a pure ![]() and that of the hybrid ``mean
field'' cation, i.e.:
and that of the hybrid ``mean
field'' cation, i.e.:
We indicate this defect as:
![]() .
.
In the same way, substitution of a mean field cation ![]() with a pure
with a pure
![]() species generates a defect with charge:
species generates a defect with charge:
which we designate as:
![]() .
.
A pure oxide ion which sits at an anionic site is seen by the mean field perspective as a defect with charge:
and thus designated as:
![]() .
.
Finally, a mean field oxide vacancy is to be indicated with
![]() .
.
In the light of the above, the mean field version of the
![]() reduction reaction can be written as follows:
reduction reaction can be written as follows:
This reaction can be decomposed, as usual, into steps whose energy change can be either directly calculated with GULP or is known from the literature:
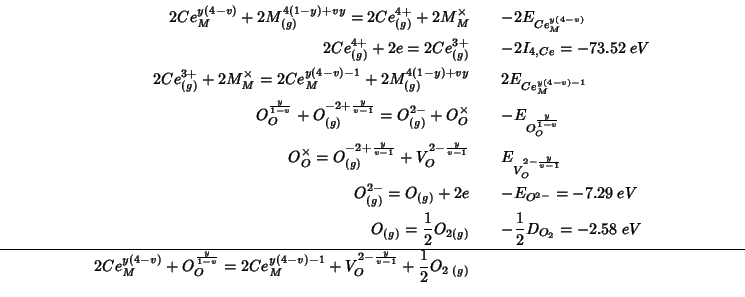
Summing up the various contributions, the
![]() reduction energy becomes:
reduction energy becomes:
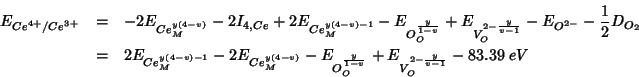
As usual, the constant terms which add up to ![]() are taken
from Table 5 of Sayle et al. (1994). The other energy contributions are
evaluated with GULP:
are taken
from Table 5 of Sayle et al. (1994). The other energy contributions are
evaluated with GULP:
| energy term | GULP code |
|
|
IMPURITY Ce4 0.00 0.00 0.00 |
|
|
IMPURITY Ce3 0.00 0.00 0.00 |
|
|
IMPURITY O 0.25 0.25 0.25 |
|
|
VACANCY O 0.25 0.25 0.25 |