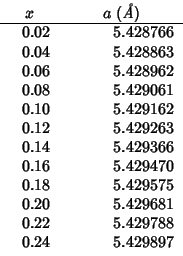
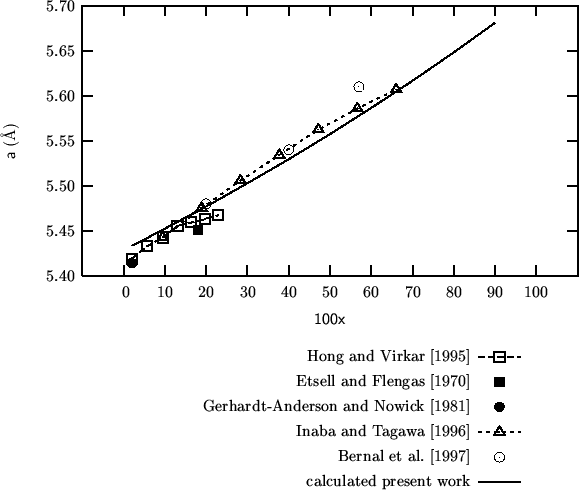
Comparison of calculated vs experimental lattice parameters for
![]() at different
compositions.
at different
compositions.
Some experimental lattice parameters for
![]() with
with
![]() can
be derived from fig. 4 of this paper.
can
be derived from fig. 4 of this paper.
A value of
![]() for the lattice parameter of
the
for the lattice parameter of
the
![]() system is quoted on p. 366. The formulation
of a binary system expressed as
system is quoted on p. 366. The formulation
of a binary system expressed as
![]() with our present
notation
with our present
notation
![]() is as
follows:
is as
follows:
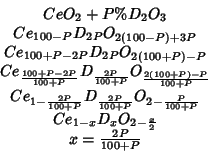
So the value quoted by Etsell and Flengas refers to the system of composition:
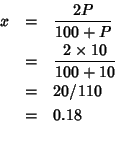
In table 1 of this paper the per cent lattice parameter change per
unit dopant concentration is reported for systems of the type:
![]() .
.
I disagree with the way data are presented because reporting the
quantity
![]() without quoting the
composition at which it was determined implicitly assumes a
linear relation between the lattice parameter and the
composition, which is not at all guaranteed. Taking the
ceria-lanthana system as an example, one could say:
without quoting the
composition at which it was determined implicitly assumes a
linear relation between the lattice parameter and the
composition, which is not at all guaranteed. Taking the
ceria-lanthana system as an example, one could say:
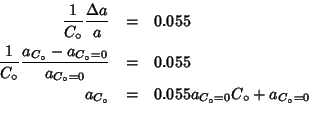
which would give the lattice parameter at any dopant
concentration once the value for pure ceria is assigned
(e.g.
![]() ).
).
Actually, this seems to have been done in figs. 7-10
of Minervini et al. (1999) for a (not so) small composition range
(
![]() for the system
for the system
![]() ).
).
However, since it appears that the data in table 1 were collected for
systems of the type
![]() (see the second
paragraph of the second column on p. 548), I think that it's more
correct to take the lattice parameters as referring to this single
composition, which corresponds to
(see the second
paragraph of the second column on p. 548), I think that it's more
correct to take the lattice parameters as referring to this single
composition, which corresponds to ![]() for the system
formulated as
for the system
formulated as
![]() .
.
Given the above, the experimental value which can be extracted from
this paper for the lattice parameter of the
![]() system is:
system is:
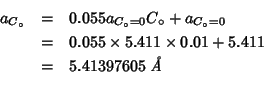
for ![]() .
.
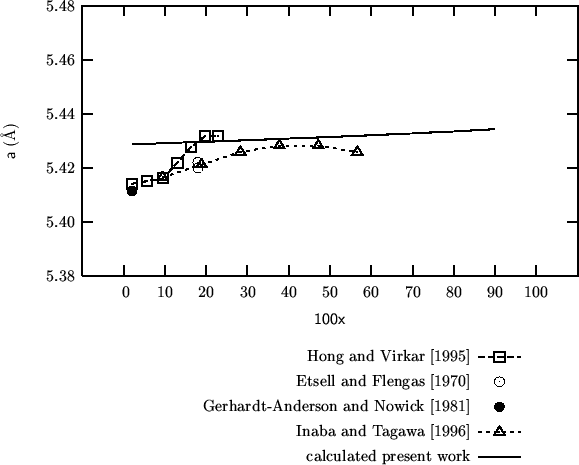
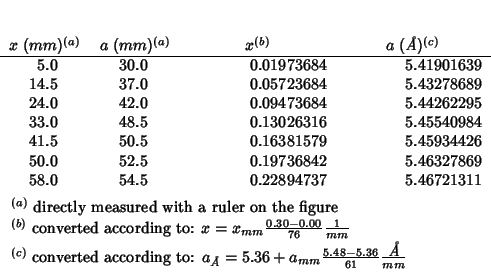
Fig. 8 of this paper reproduces the lattice parameter variation as a function of composition for a series of doped cerias from the work of Bevan and Summerville (1979). (The same work is cited by Minervini et al. (1999), but the citation seems to be different and also the data shown in fig. 7 of Grimes' paper do not look the same as those in fig. 8 of Inaba's paper: ?????)
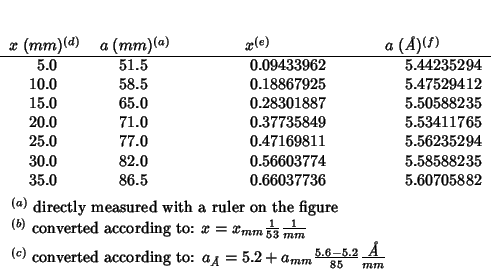

For better comparison I've evaluated some additional data in the
interval
![]() .
.
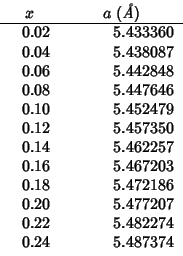
The following values are reported on p. 366 for the lattice parameter of the
![]() system (equivalent to
system (equivalent to
![]() ):
):
![]() .
.
From table 1 of this paper the following value of the lattice
parameter for the system
![]() can be
obtained (notation
and comments on this paper to be found above):
can be
obtained (notation
and comments on this paper to be found above):
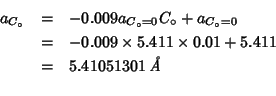
From fig. 8 of this paper (comments to be found above):


For better comparison I've evaluated some additional data in the
interval
![]() .
.
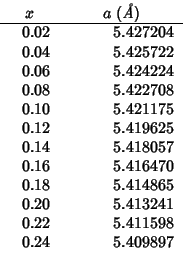
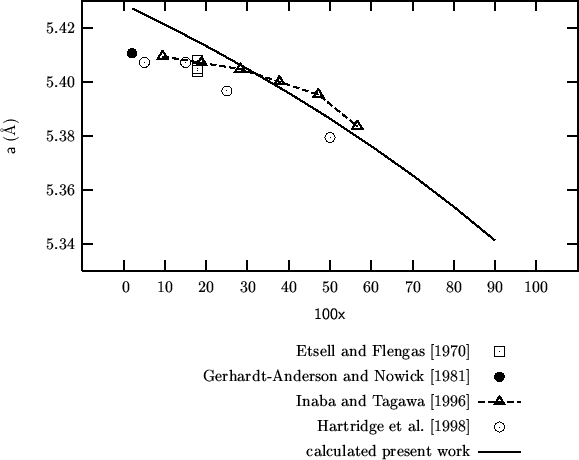
Some experimental lattice parameters for
![]() with
with
![]() can
be derived from fig. 4.
can
be derived from fig. 4.

The following values are reported on p. 366 for the lattice parameter of the
![]() system (equivalent to
system (equivalent to
![]() ):
):
![]() .
.
From table 1 of this paper the following value of the lattice
parameter for the system
![]() can be
obtained (notation
and comments on this paper to be found above):
can be
obtained (notation
and comments on this paper to be found above):
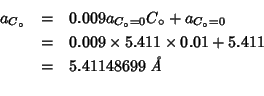
From fig. 8 of this paper (comments to be found above):

For better comparison I've evaluated some additional data in the
interval
![]() .
.