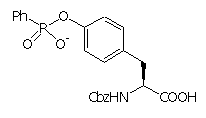
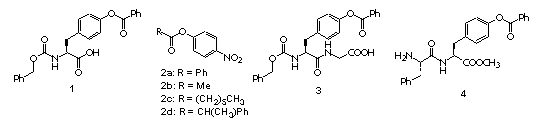
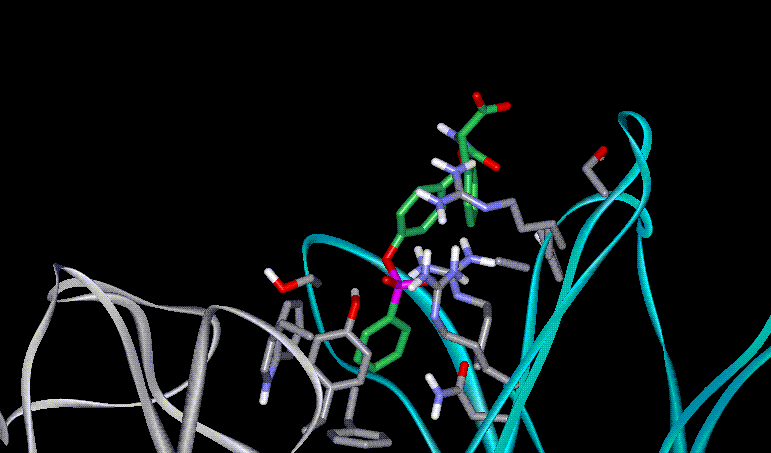
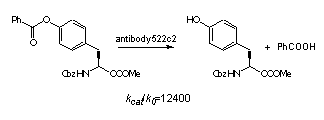The control of reactivity of functional groups on the side chains of amino acids and peptides is a potentially attractive field for applications of catalytic antibodies. The selective modification of amino acid side chains in peptides and proteins is important for many areas of chemistry, from organic synthesis to the irreversible inhibition of enzymes and the improvement of pharmacokinetic properties of peptide drugs.a However, the high level of control over chemo- and regioselectivity which is required for these transformations is not easily obtained by chemical or enzymatic approaches.b
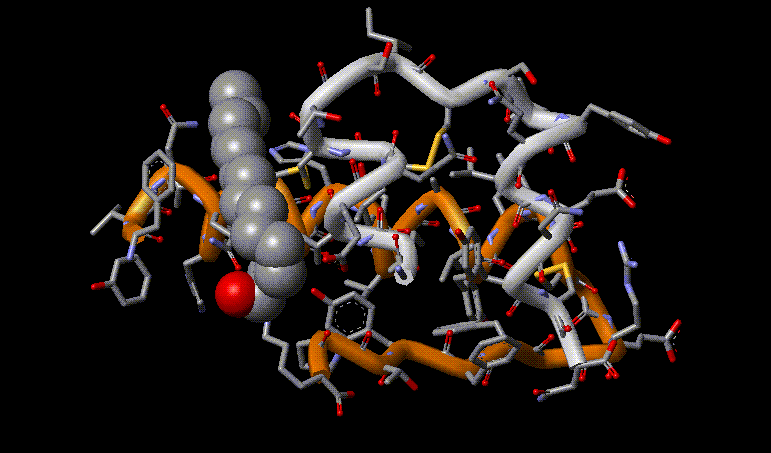
Myristoylation of insulin at a lysine residue largely improves the bioavailability of the hormone.c