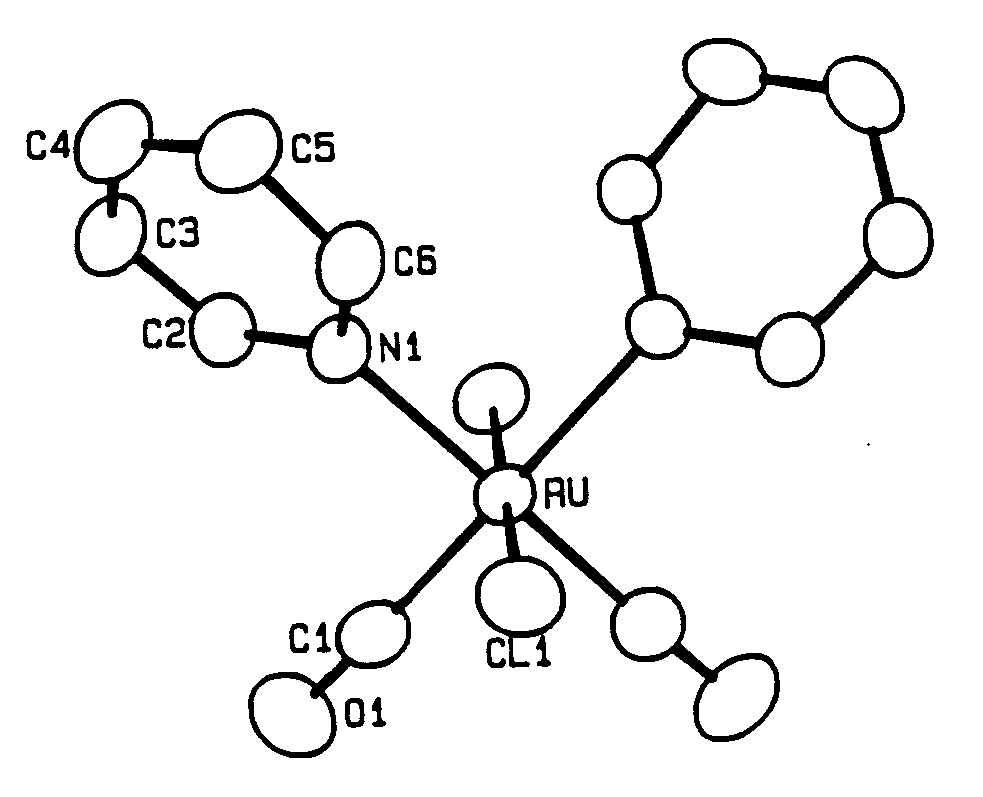
The crystal structure of cis,cis,trans-Ru(CO)2(py)2Cl2 has been determined by a single crystal X-ray analysis. Crystals are monoclinic, space group C2/c, with a = 7.426(3), b = 14.386(2), c = 13.179(6) Å,
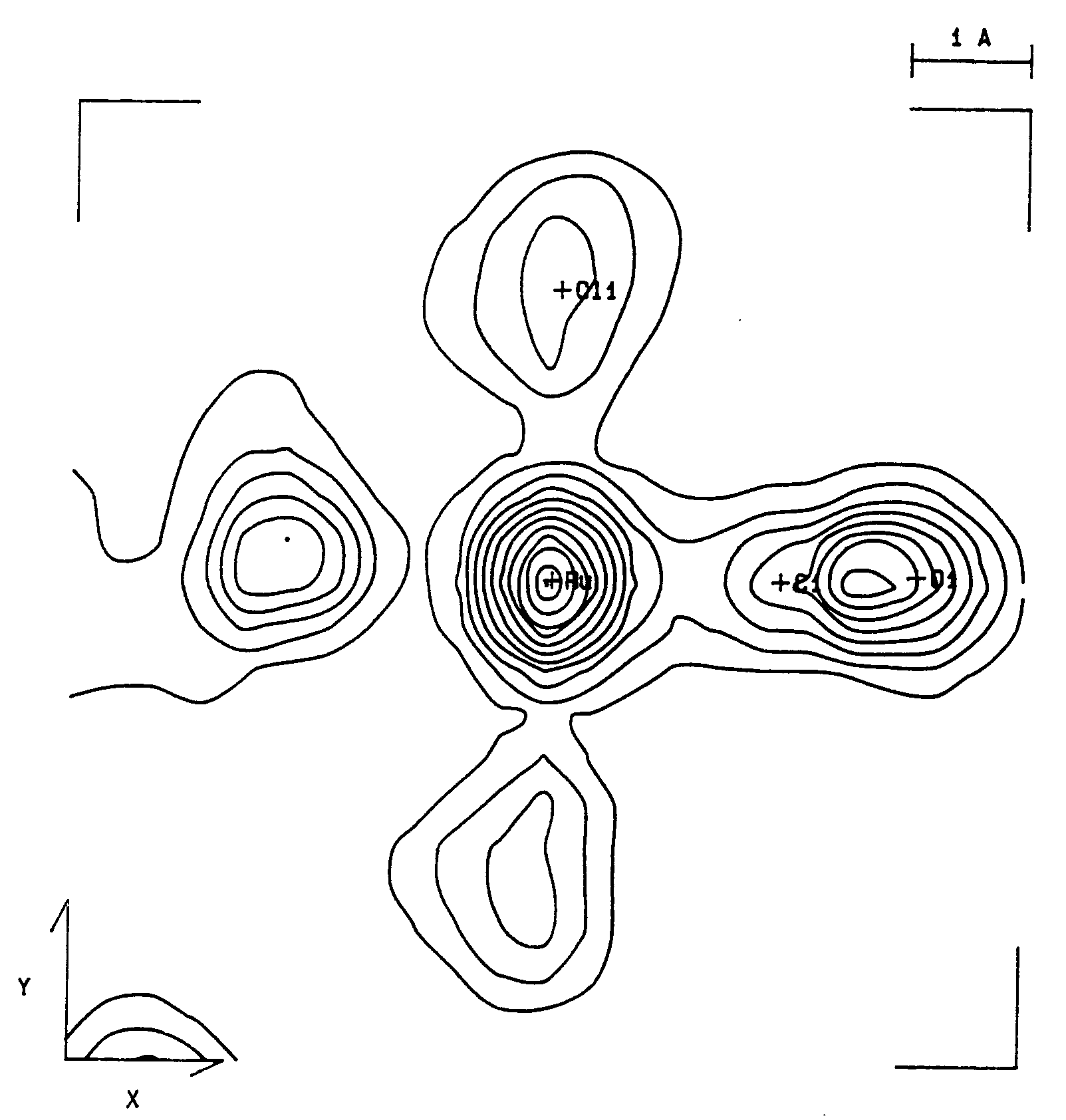
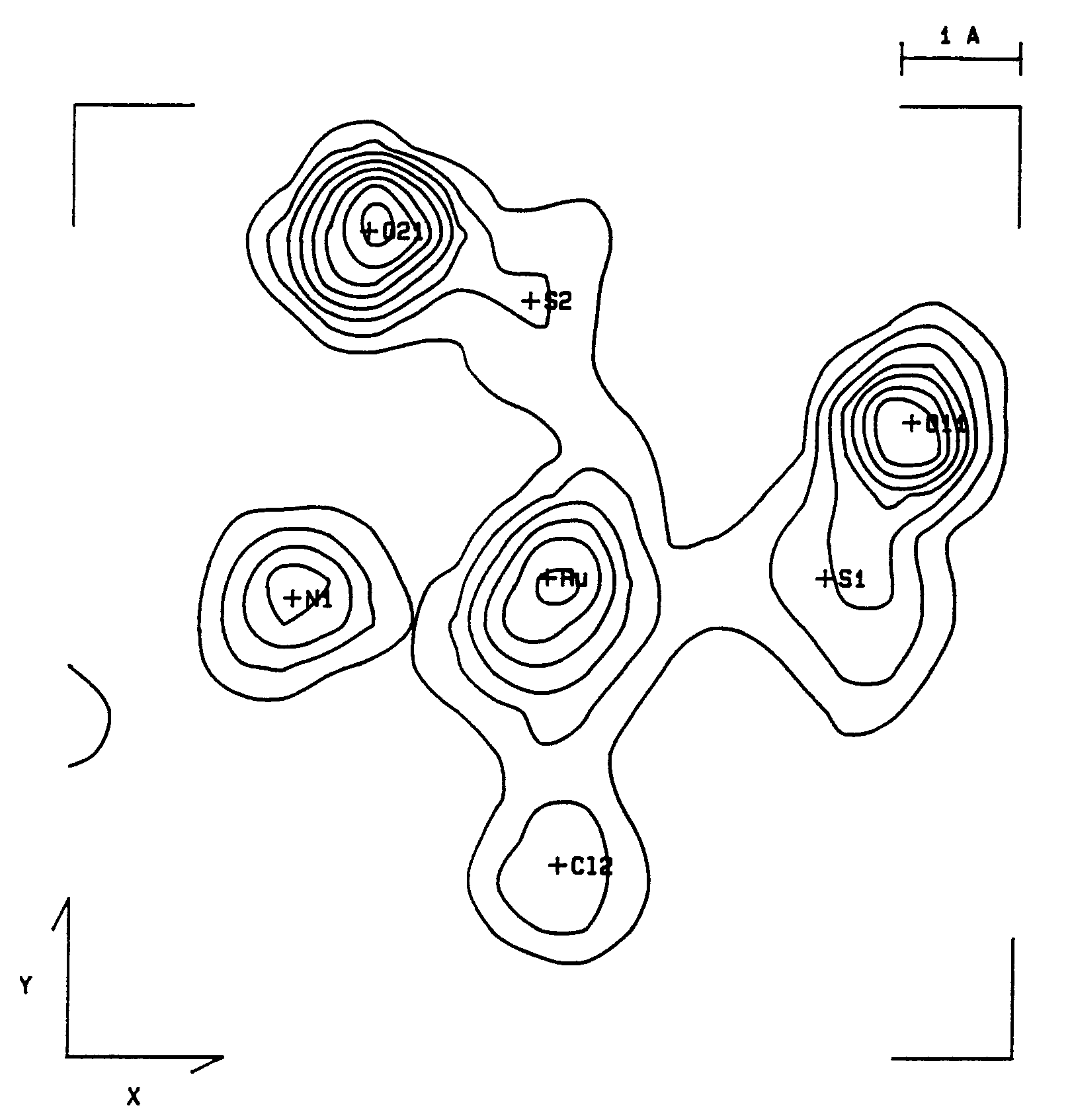
b = 91.01(2)°. Anisotropic least-squares refinement yielded R = 0.025 and Rw = 0.026. The Ru-C and C-O bond distances of 1.921(3) and 1.054(4) Å, respectively, showed to be strongly affected by bonding electron density deformation. This has been confirmed by analysis of valence electron density maps. High-order and Dunitz-Seiler refinements were used to derive correct atomic positions, which gave 1.875(5), 1.156(8) Å and 1.890(3), 1.151(5) Å, respectively, for the Ru-C and C-O bond lengths. A Dunitz-Seiler refinement was also applied to cis,cis,cis-Ru(CO)(py)(DMSO)2Cl2. Radial refinement of both structures was used to calculate net atomic charges. Negative charges are found on the carbonyl C atoms and slightly positive charges on the O atoms. For the DMSO ligand the valence electron map shows a displacement of charge from S to O atoms. The calculated charges yield S-O dipole moments in fairly good agreement with the experimental value of 3.0 D.

Fig. 1. Valence electron density maps.
A coordination plane containing the C-O bond of cis,cis,trans-Ru(CO)2(py)2Cl2
B coordination plane containing the S-O bonds of
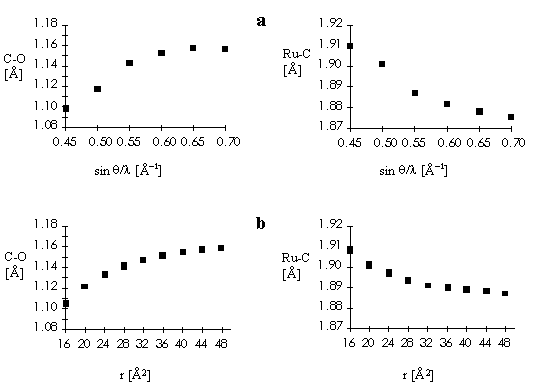
Fig. 2. Ru-C and C-O bond distances for high-order (a) and Dunitz-Seiler (b) refinements.
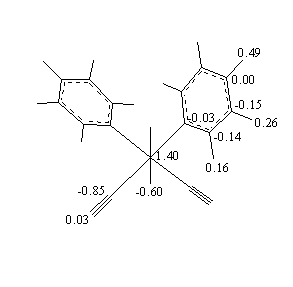
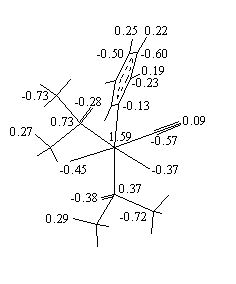
Fig. 3. Atomic charges (e) for chemically not equivalent atoms obtained from radial refinements.
![]() Structural Chemistry Group Home Page
Structural Chemistry Group Home Page